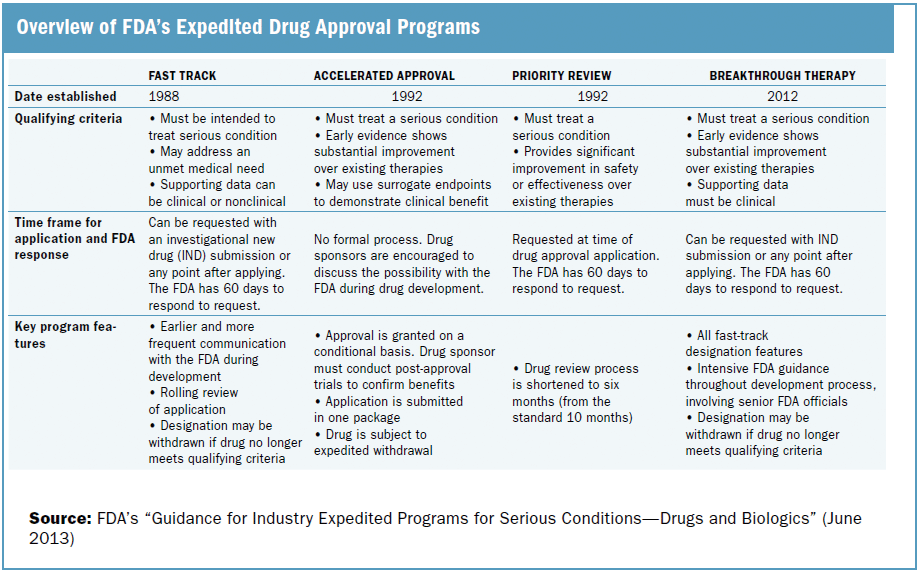
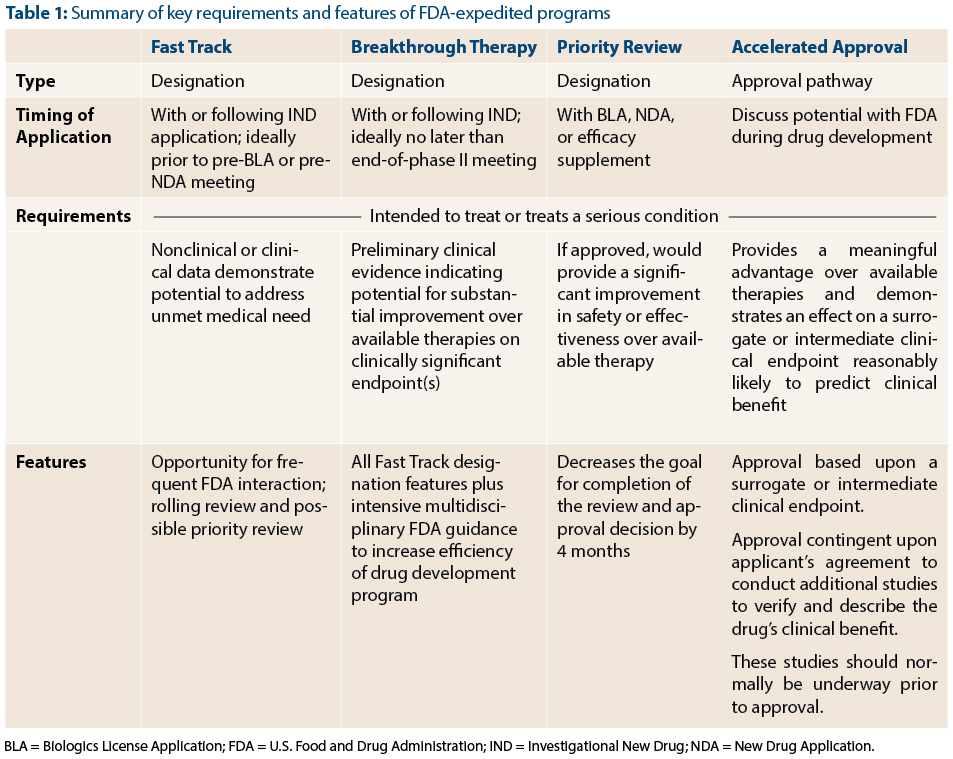
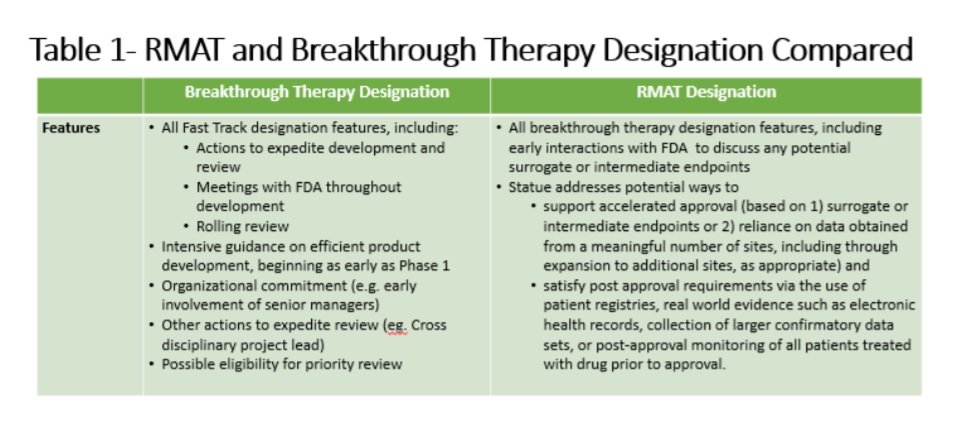
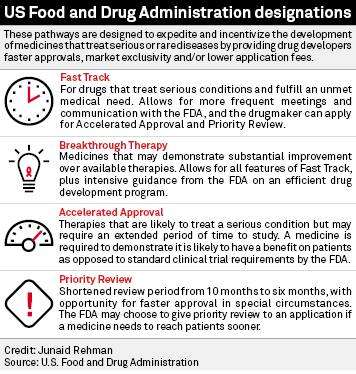
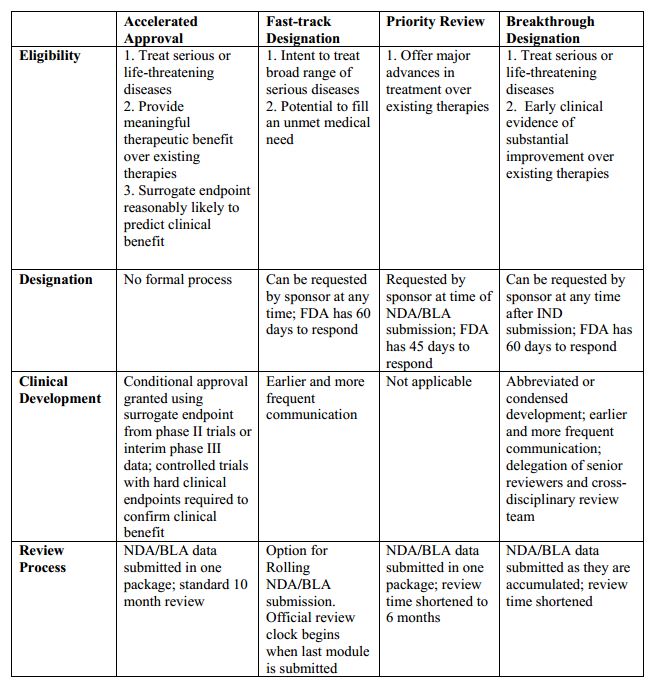
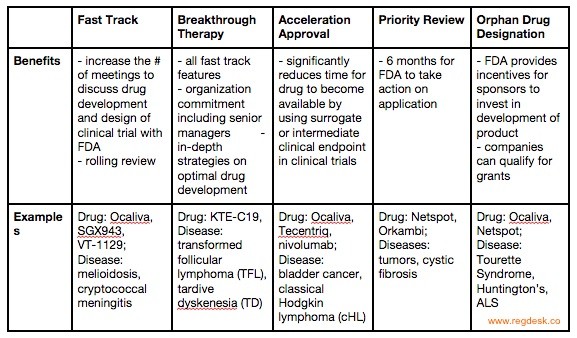
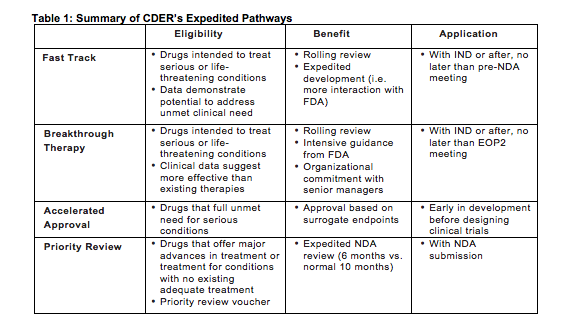
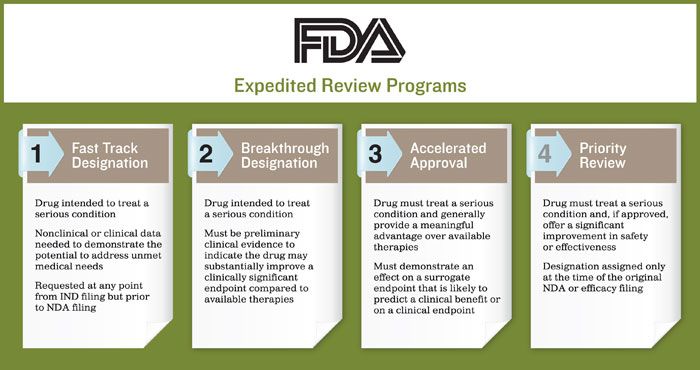
Nassau EM - What is the difference between an Emergency Use Authorization (EUA) and the Food & Drug Administration's normal medication/vaccine approval process? Before any medication can be used or prescribed in
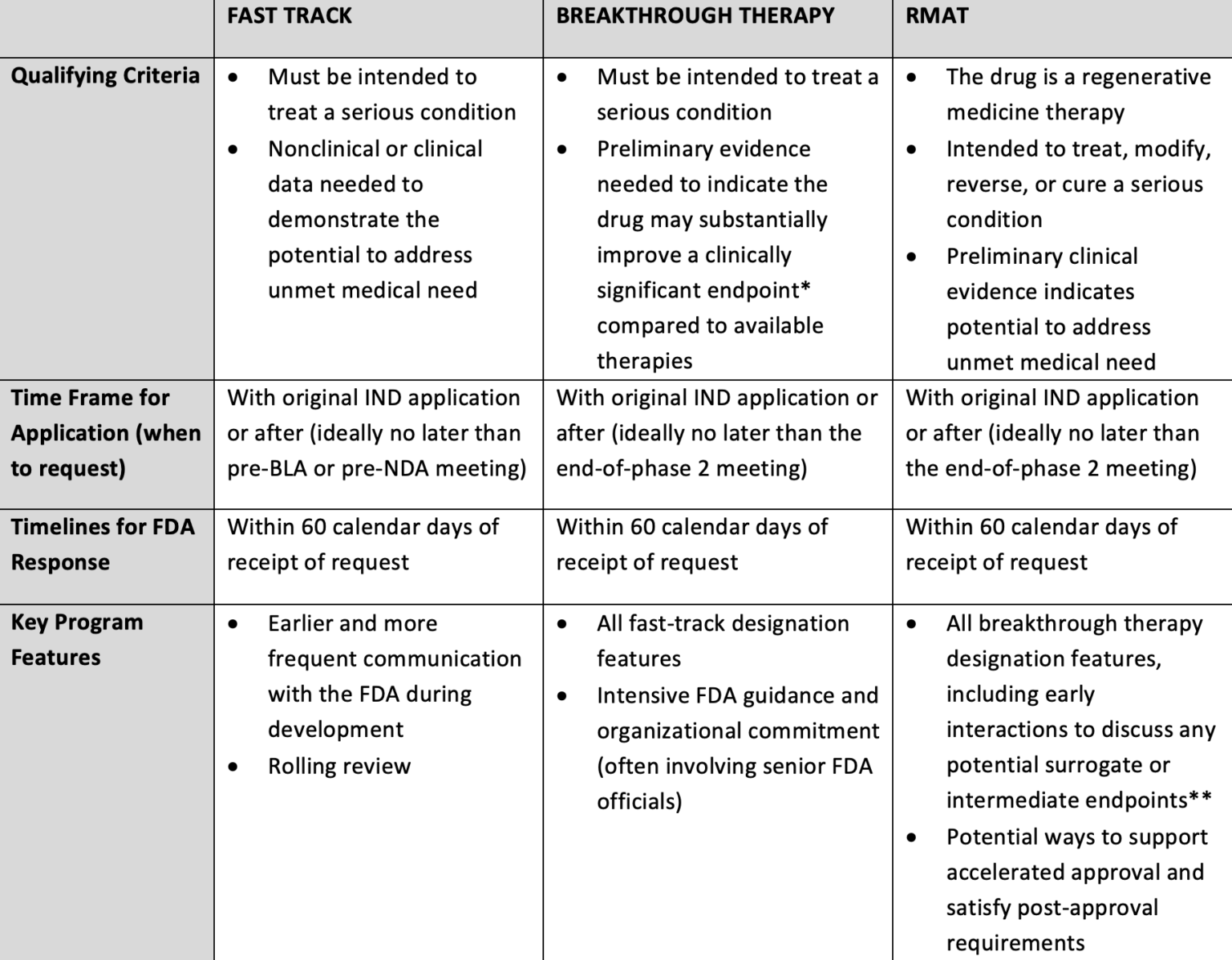
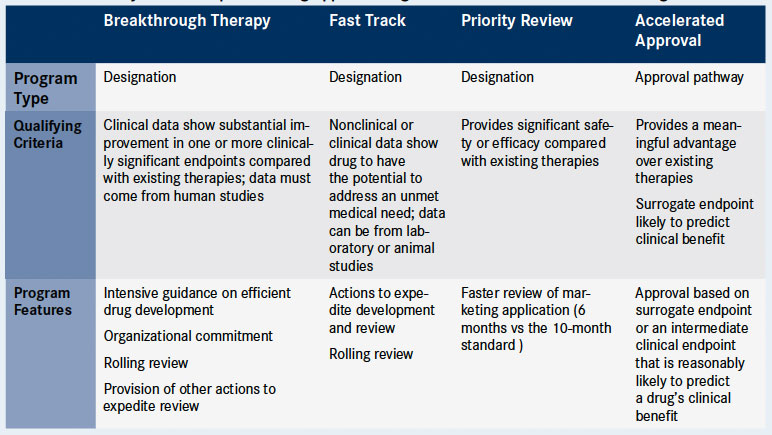
The Need for Speed in Drug Development: A Sponsor's Guide to FDA Expedited Programs | Halloran Consulting Group
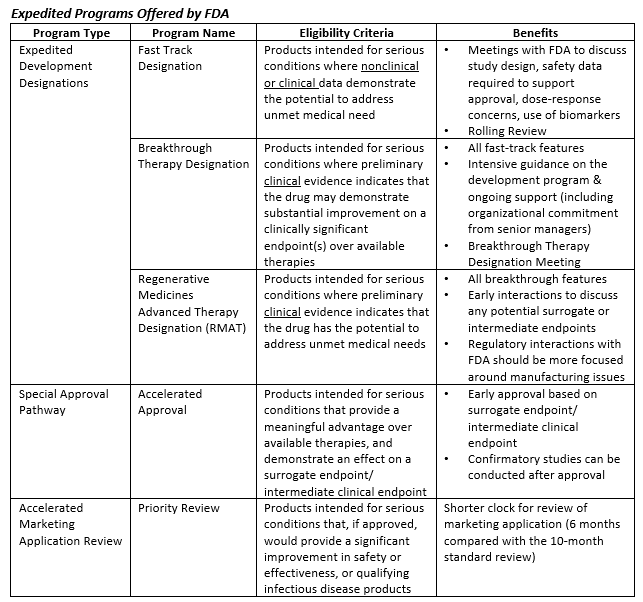
FDA efficiency for approval process of COVID-19 therapeutics | Infectious Agents and Cancer | Full Text

Regulatory Approval of Treatment for Ebola Virus: A U.S. and European Perspective - Science in the News

More Ice in the Winter Time: FDA Breakthrough Therapy Designations – Great PR While Patients Suffer — Innovation Breakdown: How the FDA and Wall Street Cripple Medical Advances by Joseph V. Gulfo